Methylation is a metabolic process that occurs in every cell and organ in our body. Life would not exist without it. Methylation occurs when one molecule passes a methyl group (a carbon atom with three hydrogens attached to it) to another molecule. This takes place around a billion times per second in our body. Methylation is critical for detoxification, immune function, DNA production, energy, mood balancing and controlling inflammation. Poor methylation function leads to many chronic conditions.
METHYLATION & DOWN SYNDROME by Gabi Giacomin
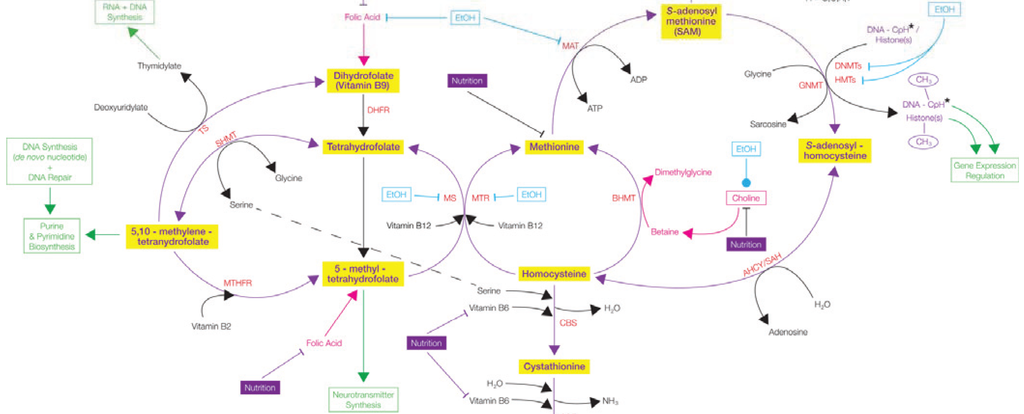
The folate cycle
Folate metabolism occurs due to several key enzymes including MTHFR which converts 5,10 mthf – 5 mthf; MTR which is involved in remethylation of homocysteine to methionine; MTRR which regenerates methionine synthase; CBS which catalyses the transulfuration of homocysteine to cystathionine and RFC-1 which transports 5-mthf into cells.[1,2,3,4,5]
Various clinical studies have found an association between MTHFR 677TT, elevated homocysteine and reduced methionine in mothers of DS children [6, 7] These results indicate that reduced MTHFR enzyme activity results in DNA hypomethylation which may lead to abnormal cell division and trisomy 21 [6]
MTHFR
At present we know a) the biochemical structure and function of the MTHFR enzyme b) the effect of both 677T and 1298C alleles of MTHFR on Homocysteine levels c) folate is not synthesised efficiently from the diet d) TT homozygotes are at risk when their folate status is low as the mutation requires much higher levels of folate to stabilise the binging of FAD e) FAD is held onto as folate levels increase [8]. Maternal nutritional status and lifestyle together with genetic mutations influence the stability of the homocysteine cycle and the production of SAMe.[8]
MTRR/ MTR & Vitamin B12
The MTRR enzyme converts cobalamin to methylcobalamin, (the active form of vitamin B12), using SAMe and B vitamins as cofactors. Mutations of MTRR, such as C524T and A66G, increase the likelihood that a person with Down Syndrome will develop Congenital Heart Defects (CHD) [9]. These polymorphisms could be used to evaluate CHD risk in DS [9] MTR influences the progression of Alzheimer’s disease [10].
Mitochondria
In DS, over expression of the CBS enzyme results in imbalances within the methylation cycle and methyl availability. Mitochondrial function is affected by methylation status, since mitochondria rely on the availability of SAMe as a methyl donor. Mitochondrial levels of SAMe are reduced in DS compared to controls indicating the effects of methylation imbalances on mitochondria [11]
Hypermethylation
Ironically, despite hypomethylation leading to low SAMe, DNA lymphocyte hypermethylation simultaneously exists in DS [12, 13] and is a potential cause of neurodegeneration [14]. Alterations in DNA methylation are associated with neurodevelopment deficits and premature ageing in DS [15, 16, 17]
Altered methylation was observed across the whole genome, and wasn’t enhanced on Chromosome 21 (HSA21) [17]. The gene DNMT3L sits on HSA21 and influences enzymes DNMT3a and 3b which are DNA methylators, and may be the cause of hypermethylation and neurological deficits in DS [17]. DNA methylation is linked to cognition in DS as measured by the Dalton Brief Praxis Test [18, 19].
CBS Enzyme
The gene for cystathionine beta-synthase (CBS) is located on chromosome 21 and is overexpressed in children with Down syndrome (DS), or trisomy 21. As a result, plasma levels of homocysteine, methionine, S-adenosylhomocysteine (SAH), and S-adenosylmethionine (SAMe) were all decreased in children with DS [20]. Levels of Cysteine and cystathionine were increased, relative to an increase in CBS activity [20].
An increase in CBS activity prevents the resynthesis of methionine from homocysteine, dependent on folate. A reduction in the availability of homocysteine creates a ‘folate trap’, resulting in folate deficiency which contributes to the pathology of DS [20].
A decrease in the activity of CBS leads to homocysteinuria, and issues with sulfur metabolism resulting in mental retardation and vascular disease [21]. Levels of CBS in DS brains are three times greater than controls and are found in astrocytes associated with plaque in the brains of DS people with AD [21]. CBS over expression may lead to cognitive decline and Alzheimer’s Disease pathology in DS [21].
Mice studies reveal CBS is over expressed in the cerebellum and hippocampus, and this over expression affects neurones in the hippocampus, facilitating the strengthening of synapses [22]. Many animal studies report that this is associated with improved spatial learning. This raises the possibility that CBS over expression might have a positive effect on cognitive function in DS [22].

SOD1
Plasma glutathione levels were reduced in children with DS possibly as a result of the over expression of the SOD gene located on chromosome 21 and the resulting increase in oxidative stress [20].
Nutrients associated with Methylation
Addition of folinic acid, methionine, methyl B12, thymidine and DMG to T21 lymphocyte cells in vitro improved the metabolic profile [20].
Homocysteine
Overexpression of CBS activity leads to low Homocysteine levels in people with DS. High homocysteine levels were found in a subgroup of people with DS who had low IQ [23]. Vitamin B12, folate and Body Mass Index weren’t related to Homocysteine levels [23]. Ageing is related to Homocysteine and IQ, although this association could not be related to DS [23]. Intelligence was associated with Homocysteine levels independent of age. The MTHFR 677T allele was associated with lower IQ due to its effect on Homocysteine levels [23]. Homocysteine and folate deficiencies increased the risk of neurodegeneration [23].
The Folate Trap
The Folate Trap in DS occurs due to the over expression of the CBS enzyme, increasing activity of the trans-sulfuration pathway. Homocysteine levels are reduced and its remethylation, dependent on 5-mthf and vitamin B12, cannot occur and neither resynthesis of 5-mthf to thf [20]. Supplementation of active folate and B12 is very effective at increasing methionine and SAMe. The MTHFR 677TT mutation may aggravate the folate trap further by reducing the remethylation of homocysteine and production of tetrahydrofolate [20]. A Mutated MTHFR also reduces production of SAMe, needed for DNA and protein methylation and synthesis of choline [20].
Maternal Risk Factors
Abnormal cell division of chromosome 21 occurs due to reduced methylation as a result of epigenetic changes [24]. Mutations of DNMT3B, a gene associated with folate metabolism, affects the activity of its enzyme and increases the chance of abnormal cell division in mothers of children with DS [24].
MTR gene mutations and homocysteine are risk factors for [Sicilian] mothers to have a child with DS [25].
Women with both MTHFR and MTRR mutations have a higher chance of producing a child with DS [26]. MTRR A66G is more common in mothers of children with DS, but isn’t associated with increased homocysteine [26].
Homocysteine levels were higher among mothers of DS children compared to controls and the homozygous MTHFR 677T allele was associated with altered levels of Homocysteine. Mothers of children with DS tended to have higher allele’s i.e. 677T, 1298C, 2756 G, 66G than controls [27].
DNA hypomethylation can lead to chromosomal instability and increase the likelihood of abnormal cell division which causes trisomy 21 [28].
Three combined genotypes (CTCC, TTAC and TTCC) were identified in Indian mothers of children with DS, but not in controls. These genotypes are very uncommon in the human population [28].
References
1. Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, Matthews RG, et al. Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification. Nat Genet 1994;7:195–200.
2. Leclerc D, Campeau E, Goyette P, Adjalla CE, Christensen B, Ross M, et al. Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum Mol Genet 1996;5:1867–74.
3. Leclerc D, Wilson A, Dumas R, Gafuik C, Song D, Watkins D, et al. Cloning and mapping of a cDNA for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proc Natl Acad Sci USA 1998;95:3059–64.
4. Butler C, Knox AJ, Bowersox J, Forbes S, Patterson D. The production of transgenic mice expressing human cystathionine beta- synthase to study Down syndrome. Behav Genet 2006;36: 429–38.
5. Nguyen TT, Dyer DL, Dunning DD, Rubin SA, Grant KE, Said HM. Human intestinal folate transport: cloning, expression, and distribution of complementary RNA. Gastroenterology 1997;112:783–91
6.James SJ, Pogribna M, Pogribny IP, Melnyk S, Hine RJ, Gibson JB, et al. Abnormal folate metabolism and mutation in the methylenetetrahydrofolate reductase gene may be maternal risk factors for Down syndrome. Am J Clin Nutr 1999;70:495–501.
7. Hobbs CA, Sherman SL, Yi P, Hopkins SE, Torfs CP, Hine RJ, et al. Polymorphisms in genes involved in folate metabolism as maternal risk factors for Down syndrome. Am J Hum Genet 2000;67:623–30.
8. Martínez-Frías.The biochemical structure and function of methylenetetrahydrofolate reductase provide the rationale to interpret the epidemiological results on the risk for infants with Down syndrome.Am J Med Genet A. 2008 Jun 1;146A(11):1477-82.
9.Ambreen Asima, Sarita Agarwala, Inusha Panigrahib. MTRR gene variants may predispose to the risk of Congenital Heart Disease in Down syndrome patients of Indian origin. Egyptian Journal of Medical Human Genetics Volume 18, Issue 1, January 2017, Pages 61–66
10. Guéant J L, Anello G, Bosco P,Guéant-Rodríguez R M, Romano A, Barone C, Gérard P, Romano C.Homocysteine and related genetic polymorphisms in Down’s syndrome IQ. J Neurol Neurosurg Psychiatry 2005;76:706-709
11. Infantino V, Castegna A, Iacobazzi F, Spera I, Scala I, Andria G, Iacobazzi V. Impairment of methyl cycle affects mitochondrial methyl availability and glutathione level in Down’s syndrome. Mol Genet Metab. 2011 Mar;102(3):378-82.
12. Kerkel, K, Schupf N, Hatta K, Pang D, Salas M, Kratz A, Minden M, Murty V, Zigman W.B, Mayeux R.P, Jenkins E.C, Torkamani A, Schork N.J, Silverman W, Croy B.A, Tycko B, 2010. Altered DNA methylation in leukocytes with trisomy 21. PLoS Genet. 6, e1001212.
13. Sailani M.R, Santoni F.A, Letourneau A, Borel C, Makrythanasis P, Hibaoui Y, Popadin K, Bonilla X, Guipponi M, Gehrig C, Vannier A, Carre-Pigeon F, Feki A, Nizetic D, Antonarakis S.E, 2015. DNA-methylation patterns in trisomy 21 using cells from monozygotic twins. PLoS One 10, e0135555.
14. Sanchez-Mut J.V, Heyn H, Vidal E, Moran S, Sayols S, Delgado-Morales R, Schultz M.D, Ansoleaga B, Garcia-Esparcia P, Pons-Espinal M, De Lagran M.M, Dopazo J, Rabano A, Avila J, Dierssen M, Lott I, Ferrer I, Ecker J.R, Esteller M, 2016. Human DNA methylomes of neurodegenerative diseases show common epigenomic patterns. Transl. Psychiatry 6, e718.
15. Bacalini M.G, Gentilini D, Boattini A, Giampieri E, Pirazzini C, Giuliani C, Fontanesi E, Scurti M, Remondini D, Capri M, Cocchi G, Ghezzo A, Del Rio A, Luiselli, D., Vitale, G., Mari, D., Castellani, G., Fraga, M., Di Blasio, A.M., Salvioli, S., Franceschi, C., Garagnani, P., 2015. Identification of a DNA methylation signature in blood cells from persons with Down Syndrome. Aging (Albany NY) 7, 82–96
16. Horvath, S., Garagnani, P., Bacalini, M.G., Pirazzini, C., Salvioli, S., Gentilini, D., Di Blasio, A.M., Giuliani, C., Tung, S., Vinters, H.V., Franceschi, C., 2015. Accelerated epigenetic aging in Down syndrome. Aging Cell 14, 491–495
17. Lu, J., Mccarter, M., Lian, G., Esposito, G., Capoccia, E., Delli-Bovi, L.C., Hecht, J., Sheen, V., 2016. Global hypermethylation in fetal cortex of Down syndrome due to DNMT3L overexpression. Hum. Mol. Genet. 25, 1714–1727.
18. Jones, M.J., Farre, P., Mcewen, L.M., Macisaac, J.L., Watt, K., Neumann, S.M., Emberly, E., Cynader, M.S., Virji-Babul, N., Kobor, M.S., 2013. Distinct DNA methylation patterns of cognitive impairment and trisomy 21 in Down syndrome. BMC Med. Genomics 6, 58.
19. Coppedè F, Bosco P, Tannorella P, Romano C, Antonucci I, Stuppia L, Romano C, Migliore L. DNMT3B promoter polymorphisms and maternal risk of birth of a child with Down syndrome. Hum Reprod. 2013 Feb;28(2):545-50. doi: 10.1093/humrep/des376. Epub 2012 Oct 18.
20. Pogribna M, Melnyk S, Pogribny I, Chango A, Yi P, James SJ. Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am J Hum Genet. 2001 Jul;69(1):88-95. Epub 2001 Jun 5.
21.Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, Kimura H. Cystathionine beta-synthase is enriched in the brains of Down’s patients. Biochem Biophys Res Commun. 2005 Dec 23;338(3):1547-50. Epub 2005 Nov 2.
22. Régnier V, Billard J-M,Gupta S, Potier B,Woerner S,Paly E,Ledru A,David S, Luilier S,Bizot J-C,Vacano G,Kraus J P,Patterson D,Kruger W D,Delabar J M,London J. Brain Phenotype of Transgenic Mice Overexpressing Cystathionine β-Synthase. Published: January 12, 2012
23. Guéant J L,Anello G, Bosco1 P, Guéant-Rodríguez R M, Romano A, Barone C, Gérard P, Romano C. Homocysteine and related genetic polymorphisms in Down’s syndrome IQ. J Neurol Neurosurg Psychiatry 2005;76:706-709
24. Jaiswal SK, Sukla KK, Kumari N, Lakhotia AR, Kumar A, Rai AK. Maternal risk for down syndrome and polymorphisms in the promoter region of the DNMT3B gene: a case-control study. Birth Defects Res A Clin Mol Teratol. 2015 Apr;103(4):299-305.
25. Bosco P, Guéant-Rodriguez RM, Anello G, Barone C, Namour F, Caraci F, Romano A, Romano C, Guéant JL. Methionine synthase (MTR) 2756 (A –> G) polymorphism, double heterozygosity methionine synthase 2756 AG/methionine synthase reductase (MTRR) 66 AG, and elevated homocysteinemia are three risk factors for having a child with Down syndrome. Am J Med Genet A. 2003 Sep 1;121A(3):219-24.
26. O’Leary VB, Parle-McDermott A, Molloy AM, Kirke PN, Johnson Z, Conley M, Scott JM, Mills JL. MTRR and MTHFR polymorphism: link to Down syndrome? Am J Med Genet. 2002 Jan 15;107(2):151-5.
27. da Silva LR, Vergani N, Galdieri Lde C, Ribeiro Porto MP, Longhitano SB, Brunoni D, D’Almeida V, Alvarez Perez AB. Relationship between polymorphisms in genes involved in homocysteine metabolism and maternal risk for Down syndrome in Brazil. Am J Med Genet A. 2005 Jun 15;135(3):263-7.
28. Dutta s, Bhowmik Das A, and Mukhopadhyay K. Risk of Down syndrome conferred by MTHFR C677T polymorphism: Ethnic variations. Indian J Hum Genet. 2007 May-Aug; 13(2): 76–77.
