What are mitochondria?
“Mitochondria are not just batteries, they are exquisitely sensitive environmental sensors – constantly tuned in to whats going on in your body, receiving input from the external as well as the internal environment; infections, toxins, inflammation, immune activation and psychological stress” – Ari Whitten
Mitochondria are traditionally described as the ‘powerhouse’ of the cell, and are responsible for cellular energy production. Basically, food intake plus aerobic respiration (oxygen) are broken down inside our cells to make ATP, or energy. Mitochondria are found in every cell in the human body which require energy to function. They produce energy for organs, and are involved in over five hundred enzymatic reactions in the body. If your mitochondria aren’t working efficiently, it effects every part of you; your muscles, gut, liver, heart, thyroid, immune system and brain are some of the most mitochondria and energy dense areas of the body. The immune system requires more mitochondrial energy than any other organ, so its no surprise that when our immune system is under attack from an infection (ie. common cold), the body diverts its energy production to immune cell defense at the expense of everything else (and we feel tired).
New Insights into Mitochondria
In an acute stress situation, mitochondria divert ATP (energy) production to immune support and afterwards, stimulate anti-inflammatory, regenerative pathways to restore healing. When the body experiences chronic stress from infections, toxins, heavy metals, poor sleep, emotional stress, poor circadian rhythm, EMFs, etc the diversion of energy production towards immune support continues abnormally (Cell Danger Response) and fatigue sets in as mitochondria divert activity away from ATP production (energy). This diversion of energy production to chronic immune defense, is now recognised as the cause of autoimmunity, neurological and developmental disorders as the ability of organs to maintain energy and efficient function become compromised.
Read more about the Cell Danger Response and Dr Robert Naviaux Here!
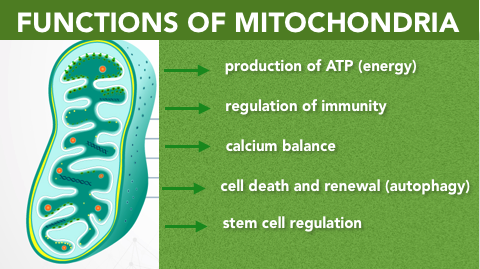
Figure 1. The 5 Main Functions of Mitochondria; energy production, regulation of immunity, calcium balance, mitophagy, stem cell regulation.
DS Genes + Mitochondria
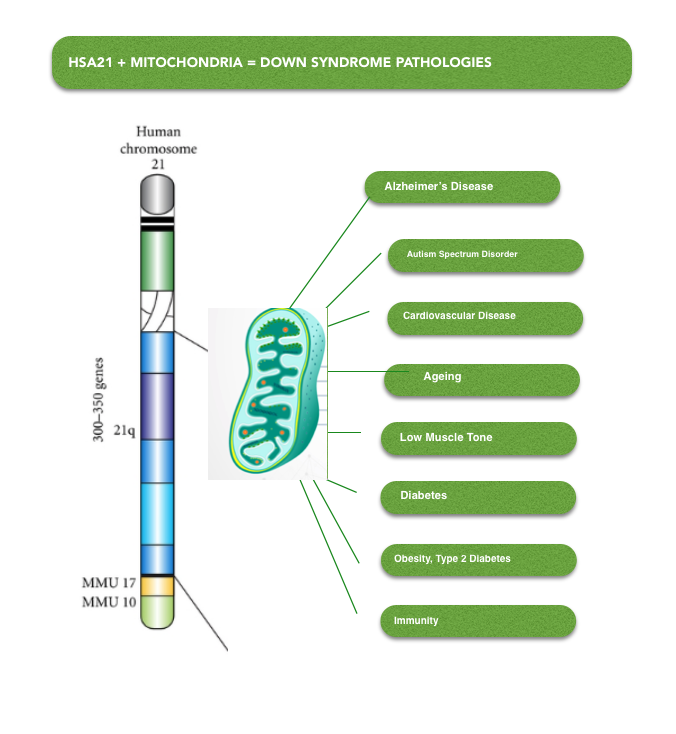
In Down Syndrome (DS) mitochondria are functioning sub-optimally due to the over-expression of genes on Chromosome 21 (Hsa21). Hsa21 genes reduce the activity of genes and enzymes which regulate mitochondrial structure and function. This is recognised as a primary cause of intellectual disability and neurodegerative progression to Azheimers Disease (AD) in DS. In addition, poor muscle tone, heart defects, type 2 diabetes, obesity, immune disorders and autistic traits are also symptoms of mitochondrial dysfunction in DS.
The diagram below (Figure 2) illustrates the activity of genes located on Hsa21 on mitochondria. Genes located on Hsa 21 (blue boxes) reduce activity of mitochondrial genes (yellow boxes) due to their overexpression. This leads to alterations in mitochondrial structure and function and the inability to produce ATP efficiently (orange boxes). Increased oxidative stress and low energy are a byproduct of poor mitochondrial function. Outcomes for people with DS (grey box) include defective neurogenesis (reduced ability to form new brain cells), altered neural plasticity (ability of brain cells to change form and function in response to their environment), cognitive impairment (poor learning, concentration, memory etc) early aging and early neurodegeneration (loss of structure or function of neurons).
Figure 2: Overview of the targets of gene over-expression in Down Syndrome. (Valenti et al. Mitochondria as Pharmacological Targets in Down Syndrome, Free Radic Biol Med 2018;114:69‐83)
Mitochondria + Down Syndrome Pathology
Figure 3. Chromosome 21 impacts mitochondrial function by reducing the expression of genes associated with healthy mitochondrial form and function causing Down Syndrome pathologies to develop.
Alzheimer’s Disease
Overexpression of the APP gene located on Hsa21 (see figure 2) is thought to be responsible for the formation of amyloid plaque and the development of Alzheimer’s Disease (AD) like symptoms in DS after age 55. Poor mitochondrial function and high levels of oxidative stress are major causes of neurodegeneration and development of AD in DS. The accumulation of plaque, reduces the production of energy within mitochondria as well as the rate mitochondria consume oxygen.
Activity of PGC-1a, NRF-1, NRF-2 and TFAM (see Figure 2), (genes which regulate the growth of new mitochondria), are all reduced in the brains of people with DS due to gene overexpression, and are also found in people with AD. In addition, changes to the structure and dynamics of mitochondria were also observed in DS/ AD. When mitochondria are damaged they produce excess amounts of oxidative stress and this further damages mitochondrial DNA (mtDNA) and important enzymes.
Autism Spectrum Disorder
The development of Autism Spectrum Disorder (ASD) in children with DS is characterised by poor social skills, restricted and repetitive behaviour. Underlying genetics combined with environmental exposure are thought to manifest symptoms of ASD such as gastrointestinal issues, epilepsy, sleep disorders, ADD, ADHD, reduced motor co-ordination, intellectual disability and inattentiveness.
Mitochondrial dysfunction is now thought to play a role in the development of ASD with between 50-80% of children with autism displaying mitochondrial dysfuction. Urine mitochondrial metabolites show abnormalities in lactate and pyruvate (glycolysis – ATP cycle), as well as imbalances in aspartate, glutamate and calcium balance (regulate glutamate and GABA production in the brain).
Enzymes located in the Krebs cycle (energy production) of children with ASD are low in both brain and muscle tissue. Intracullular glutathione levels are low indicating high levels of oxidative stress linked to poor mitochondrial function.
Genes associated with disrupted mitochondrial function such as MT-ND5, ATP6, were found in mtDNA analysis of children with ASD. The NDUFS4 gene which regulates a portion of the ATP (energy) production pathway showed reduced activity in cell cultures.
Cardiovascular Disease
The role of mitochondrial dysfunction and oxidative stress in cardiovascular disease and congenital heart defects in DS is well established. Alterations in mitochondrial function have widespread effects on DS heart tissue including poor development and poor stem cell regulation, increased susceptibility to injury and congestive heart failure.
Reduced activity of the gene OPA1 (regulates mitochondrial mitophagy – programmed cell death) is linked to heart failure in DS, and this is caused by Hsa21 gene overexpression (see Figure 2. Mnf-2). OPA1 is linked to fragmented mitochondria which alters cardiac metabolism. AMPK, regulator of mitophagy (programmed cell death) is downregulated in DS. This has the effect of reducing the activity of Sirt1 and PGC-1a, both necessary for mitochondrial activity with effects on cardiovascular function.
Poor mitochondrial function increases oxidative stress, a major cause of heart disease, though other metabolic pathways are thought to contribute. MtDNA are vulnerable to oxidative stress which it generates, lacking an efficient defense and repair system. Congenital heart disease is often found along with MtDNA mutations. In heart disease, oxidative stress and reduced antioxidant protection lead to further damage to mitochondria. Stimulating AMPK, mitophagy (programmed cell death) and mitochondrial fission (division)(see EGCg below) could reduce cardiovascular complications.
Ageing
People with DS are more likely to age faster than their peers. Poor mitochondrial function and oxidative stress cause damage to cells and tissues known as the ‘free radical theory of ageing’. This occurs as a result of an imbalance between the generation of oxidative stress and the antioxidant defense network. Oxidative stress is important for maintaining neuronal function, learning, memory and the immune system, but chronic production of free radicals causes damage to important cellular processes.
The imbalance between free radical generation and reduced antioxidant status which occurs in ageing, includes reduced glutathione levels, and reduced mitochondrial activity. Oxidative stress reduces mitochondrial cAMP (intracellular signalling) levels which leads to reduced Sirt3 (longevity gene) and PKA (regulates glucose, sugar and fat metabolism) activity observed in DS patients.
Read about how Mitochondria affect Low Muscle Tone, Obesity, Type 2 Diabetes and Immunity in Down Syndrome Click Here!
Nutrigenomic Intervention restores Mitochondrial function in DS
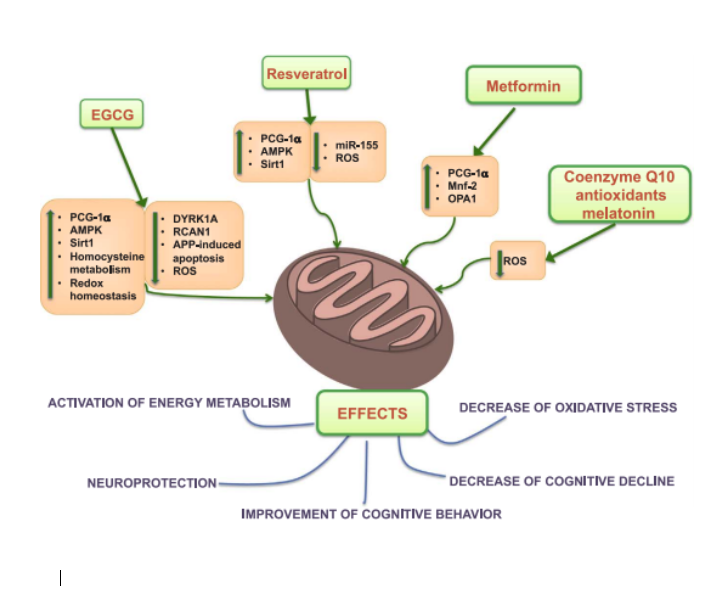
Figure 4: Overview of therapeutic interventions EGCg, Resveratrol, Metformin, CoQ10, Melatonin and their biological targets shown to reverse mitochondrial dysfunction, oxidative stress as well as some clinical symptoms of DS. (Valenti et al. Mitochondria as Pharmacological Targets in Down Syndrome, Free Radic Biol Med 2018;114:69‐83)
Targeting affected pathways is a potentially new way to restore mitochondrial function in DS. Research from the past decade points towards the use of Polyphenols and Nutrients as effective regulators of these pathways. The goal of Nutrigenomic Intervention is to reduce mitochondrial damage and oxidative stress in DS, and this is thought to be an effective tool to support the health and development of people with DS.
Polyphenols are plant derived micronutrients associated with reduced risk of chronic degenerative disease in humans. In DS they down-regulate gene expression of DYRK1A, RCAN1, miRNA-155, (see figure 4) reduce damage to cellular membranes, restore mitochondrial function and lower oxidative stress levels. In addition research shows that Polyphenols support liver function, blood glucose metabolism, protect the brain from damage via seizure activity, reduce AML Leukaemia cell activity and protect DNA from damage. Polyphenols which selectively target genes causing mitochondrial dysfunction in DS are EGCg, Resveratrol and Curcumin.
EGCg
EGCg is a catechin and the main polyphenol found in Green Tea. Its has multiple therapeutic actions on mitochondria in DS, including the ability to regulate mitochondrial activity, reduce glutamate toxicity, prevent damage to mitochondria from Aß plaque formation, maintain balanced mitochondria and reduce oxidative stress.
EGCg increases AMPK (mitophagy) levels which are low in DS and prevent proper programmed cell death and renewal. An increase in AMPK activity is associated with increased mitochondrial production of energy (ATP) as well as an increase in neurones in the hippocampus. The upregulation of AMPK by EGCg leads to activation of Sirt1, regulator of longevity, genetic stability and neurodegeneration. In addition it increases activity of PGC1a, improving mitochondrial growth and cardiovascular function.
EGCg improves activity of cAMP, PKA and NDUFS which we spoke about earlier in relation to mitochondria, ageing and ASD. Increasing the activity of this pathway saw improvements in energy production and reduced oxidative stress within the mitochondria.
EGCg down-regulates activity of DYRK1A which is overexpressed in DS and responsible for cognitive issues and poor brain development in DS. In addition EGCg reduced intermittent hypoxia affecting neurons during sleep due to oxidative stress and inflammation, improving recurrent sleep apnea.
Double blind, placebo controlled studies by de la Torre and Vacca et al, demonstrate the beneficial effect of EGCg on learning and memory, mitochondrial activity and improved behaviour in young people with DS.
Resveratrol
Resveratrol is a polyphenol found in wine, grapes, and the herb Polygonum cuspidatum. It targets the PGC1a/ Sirt1/ AMPK pathway in DS restoring mitochondrial activity in vitro and the growth of brain cells. Resveratrol also down regulates a miRNA-155 which is over expressed in DS and TFAM restoring mitochondrial activity and other altered metabolic activity. MiRNA overexpression negatively effects the blood brain barrier during neuroinflammation in DS, while Resveratrol restores it.
It should be noted that the effectiveness of polyphenols for down-regulating genes in DS is dose dependant. Specific low doses were found to be beneficial, while higher doses were harmful. The quality of the Polyphenol is also important for it to work properly.
CoEnzyme Q10
CoQ10 is directly involved in the electron transport chain during energy production. In addition it protects cellular mitochondrial structures and reduces oxidative stress. Studies of children with DS report reduced levels of CoQ10 compared to typical children and this was correlated with decreased IQ. Prolonged supplementation of CoQ10 to people with DS shows an increase in DNA repair processes.
Melatonin
Melatonin, a hormone associated with the sleep/ wake cycle showed low activity in children with DS compared to controls. In DS mice, melatonin reduced age related damage to neurons, improved learning and memory and reduced oxidative damage.
Pyrroloquinoline Quinone (PQQ)
This is a powerful co-enzyme that stimulates the growth of new mitochondria and protects the mitochondria from damage. PQQ directly targets PGC1a restoring mitochondrial activity.
Conclusion
Altered gene expression which drives mitochondrial imbalances in DS is common to other neurodegenerative diseases, ageing, heart defects, metabolic issues. The brain, being vulnerable to oxidative stress and energy deficits is primarily affected by mitochondrial dysfunction. The cognitive decline and intellectual disability seen in DS is primarily driven by mitochondrial imbalances.
Early childhood is the ideal time to implement therapeutic intervention, reducing oxidative stress and improving mitochondrial function to improve behavioural outcomes. Long term treatment is recommended since short term interventions failed to remain effective in adulthood. Polyphenols such as EGCg and Resveratrol, due to their efficacy and safety profile are strongly recommended. Combination therapy is also recommended.
Available evidence suggests improving mitochondrial function not only improves cognition but many other diseases associated with DS leading to improved health and wellbeing in this population.
How to further Optimise Mitochondrial Function Click Here!