Without targeting the over expression of genes affecting DS biochemistry, the disease process can’t be halted and regeneration of neurons will be unsuccessful. Treating a person with DS with the same approach as a non-DS person, will not improve their symptoms. Rebalancing of biochemistry must be the initial treatment approach.
Nutrigenetic Intervention by Gabi Giacomin
The aim of Nutrigenetic Intervention in Down Syndrome (DS) is to balance an individuals biochemistry by supporting pathways that are deficient and down-regulating pathways which are over-active and interfering with biochemical processes. One way to achieve this is by inhibiting the over expression of disruptive genes, which involves blocking their transcription – the first step of gene expression where a segment of DNA is copied into RNA, or increasing biochemical activity, which involves adding nutritional and phytotherapeutic supplements to augment a pathway.
An example is the over-expression of the enzyme SOD1, which places people with DS under oxidative stress as a result of increased levels of free radicals in organs, particularly the brain. ROS (reactive oxygen species) are associated with premature ageing in DS, and supplementing with specific antioxidants such as A,C and E becomes imperative if the cell is to function successfully in a oxygen rich environment [1, 2].
In addition, supplementation with Selenium and Vitamin D3 up regulates glutathione peroxidase, which detoxifies hydrogen peroxide – a byproduct of high SOD1 expression, to balance the antioxidant system [3].
Alpha-ketoglutarate, bioflavonoids, vitamin C and proline manipulate the COLVIa gene strengthening connective tissue and formation of cartilage and bone, and improving muscle tone in DS [4].
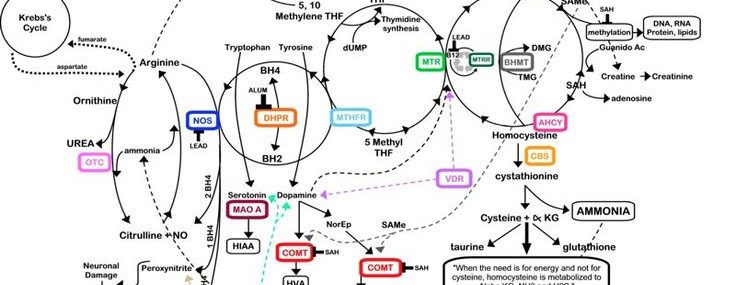
Over expression of the CBS gene leads to chronic folate deficiency resulting in poor growth, impaired adrenal sufficiency, poor purine synthesis, poor cognition and, in rare cases, Leukaemia. Supplementation with methylfolate, folinic acid, Vitamin B6 and B12 solve the problem of folate trapping caused by over expression of the CBS gene [5] Recently, a novel bioflavonoid Apigenin was introduced to the TNI protocol to inhibit the activity of the CBS enzyme.
RCAN1 is over expressed in DS and interferes with normal brain development [6]. The bioflavonoid Quercetin, antioxidant pigment Lycopene and fish oils are used as inhibitors to down regulate its function [7, 8, 9].
EGCg, down regulates DYRK1 expression to enhance cognitive ability in DS [10]. EGCg, curcumin, quercetin and resveratrol reduce IL-2 and T lymphocyte proliferation reducing the development of Autoimmunity [10a]. EGCg corrects skeletal defects in a DD mouse model [10b]. Craniofacial structure is altered in DS due to triplication of DYRK1a, significantly affecting breathing, eating and speaking. EGCg, a DYRK1a inhibitor, normalised some craniofacial characteristics when given prenatally [10c]. In a landmark study by de la Torre, EGCg reversed cognitive deficits in DS improving memory recognition, working memory and quality of life by reducing the activity of DYRK1a [10d].
PQQ up regulates CREB which down regulates RCAN1 [11]. PQQ suppresses peroxynitrite, a toxin which fuses with TAU and amyloid proteins to form Alzheimer’s plaque. It also acts as a SOD1 mimic scavenging SOD before it transforms into hydrogen peroxide [12].
MicroRNA-155 is over expressed in DS [13]. Excess microRNA-155 increases inflammation in the body resulting in a leaky blood brain barrier, leaky gut, autoimmune disease, immune deficiency and is part of the cause of Leukaemia in DS. It also plays a role in cognitive decline. MicroRNA regulates MECP a protein found to be low in autism and DS. Low levels of MECP result in damage to sensory and auditory function as message conduction between neurones is difficult. Resveratrol down regulates microRNA-155, and increases MECP [14].
Resveratrol also upregulates telomerase and facilitates the repair of telomeres [15]. Telomeres are the tips of chromosomes. One of their functions is to prevent the destruction of DNA. During cell division telomeres shorten, and over time they the become too short to sustain life. This may be a cause of reduced life expectancy in DS [15]. Resveratrol plays a critical role in increasing the life span of people with DS [15, 16].
Curcumin inhibits inflammatory cytokines which destroy neurons in the brain. It also reduces neuro inflammation to safe levels [17]. It inhibits APP and APOE proteins which are unregulated in DS and contribute to plaque formation in DS brains leading to Alzheimer’s disease [18]. Curcumin also inhibit’s microRNA-128b which is over expressed in DS and a cause of Leukaemia among other diseases.
DHA is the brains primary fatty acid. It builds myelin, the protective sheath covering neurons and assists in removal of heavy metals and other neurotoxins from the brain.

Nutrigenomics & Down Syndrome
Nutrigenomics is a scientific study which looks at the interaction of nutrition and genes, particularly in disease treatment and prevention. As nutrigenomics becomes better understood, it will improve nutritional advice to the general public, genetic subgroups and individuals with genetic disorders such as Down Syndrome (DS).
Many factors are involved in the study of nutrigenomics; the effect of diet and nutrition on 1) genetic stability, 2) changes in the genes which affect DNA methylation 3) RNA and microRNA expression 4) protein expression and 5) metabolic changes. These can all be studied to diagnose health or disease outcomes [19].
A large number of studies have clearly shown that nutrition alters the expression of our genes. Diet can affect the expression levels of genes by causing epigenetic changes such as methylating DNA. Nutritional experts are learning to analyse genetic information and influences on genes such as diet, lifestyle and environment, in order to develop nutritional programmes based on gene types, by looking at SNPs and lifestyle [19].
Advances in pathology tests which assess DNA damage have made it possible to determine nutrient reference ranges for DNA damage. Nutritional programmes are then developed to target prevention and translate this into practice [22, 23, 24]. Damage to the genome is the main cause of developmental and degenerative diseases. This can now be accurately diagnosed and prevented with appropriate diet and lifestyle intervention particularly for genetic subgroups such as people with DS [19].
The effect of food and nutrition on physiology depends on many complex processes including absorption, transport, binding, storage and excretion as well as cellular activities [19]. All of these processes can involve genes and their SNPs that can alter their activity and the physiological response to food. Genes can also affect food preferences by affecting sensory, reward or energy producing pathways [25]. Growing interest in the gut microflora and its relationship with the genome, adds another level of complexity nutrigenomic analysis.
Recommended Dietary Allowance’s
Recommended dietary allowance’s (RDA’s) have been designed for the general population without consideration for genetic subgroups who may have very different nutritional requirements such as Down Syndrome. The goal of Nutrigenomics is to align nutrient intake with genetic individuality. This allows gene expression, maintenance, metabolism and cell function to occur normally and sustains homeostasis [26, 27, 28, 29, 30, 31, 32].
Nutrigenomic research presents us with a lot of new knowledge. Particular attention needs to be given to specific genetic subgroups such as DS when formulating nutrition plans. Individual responses to dietary changes, even within genetic subgroups, can vary making it necessary to combine nutrigenomic advice with individual testing. In this way we can see whether recommended nutrients produce the expected nutritional change and health benefit within an individual.
Support
Significant scientific evidence exists to support the use of nutritional supplements in people with Down syndrome. However, it’s important to consult a healthcare professional, such as a Doctor or Naturopath with knowledge and experience of the unique physiology of a person with Down Syndrome, before starting supplementation.
References
1. El-Bassyouni HT, Afifi H, Eid MM, Kamal RM, El-Gebali HH, El-Saeed G, Thomas MM, Abdel-Maksoud SA. Oxidative Stress -a Phenotypic Hallmark of Fanconi Anemia and Down Syndrome: The Effect of Antioxidants. Ann Med Health Sci Res. 2015 May-Jun;5(3):205-12.
2. Muchová J, Žitňanová I, Ďuračková Z. Oxidative stress and Down syndrome. Do antioxidants play a role in therapy? Physiol Res. 2014;63(5):535-42. Epub 2014 Jun 5.
3.Tekşen F, Sayli BS, Aydin A, Sayal A, Işimer A. Antioxidative metabolism in Down syndrome. Biol Trace Elem Res. 1998 Aug;63(2):123-7.
4. Dey A, Bhowmik K, Chatterjee A, Chakrabarty PB, Sinha S, Mukhopadhyay K. Down Syndrome Related Muscle Hypotonia: Association with COL6A3 Functional SNP rs2270669. Front Genet. 2013 Apr 22;4:57.
5. Fillon-Emery N, Chango A, Mircher C, Barbé F, Bléhaut H, Herbeth B, Rosenblatt DS, Réthoré MO, Lambert D, Nicolas JP. Homocysteine concentrations in adults with trisomy 21: effect of B vitamins and genetic polymorphisms. Am J Clin Nutr. 2004 Dec;80(6):1551-7.
6. Dashinimaev EB, Artyuhov AS, Bolshakov AP, Vorotelyak EA, Vasiliev AV. Neurons Derived from Induced Pluripotent Stem Cells of Patients with Down Syndrome Reproduce Early Stages of Alzheimer’s Disease Type Pathology in vitro. J Alzheimers Dis. 2017;56(2):835-847.
7. Lim S, Hwang S, Yu JH, Lim JW, Kim H. Lycopene inhibits regulator of calcineurin 1-mediated apoptosis by reducing oxidative stress and down-regulating Nucling in neuronal cells.Mol Nutr Food Res. 2016 Dec 8.
8. Zmijewski PA, Gao LY, Saxena AR, Chavannes NK, Hushmendy SF, Bhoiwala DL, Crawford DR.
Fish oil improves gene targets of Down syndrome in C57BL and BALB/c mice. Nutr Res. 2015 May;35(5):440-8.
9. Zhao Y, Zhang J, Shi X, Li J, Wang R, Song R, Wei Q, Cai H2, Luo J. Quercetin targets the interaction of calcineurin with LxVP-type motifs in immunosuppression. Biochimie. 2016 Aug;127:50-8.
10. De la Torre R, De Sola S, Pons M, Duchon A, de Lagran MM, Farré M, Fitó M, Benejam B, Langohr K, Rodriguez J, Pujadas M, Bizot JC, Cuenca A, Janel N, Catuara S, Covas MI, Blehaut H, Herault Y, Delabar JM, Dierssen M. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res. 2014 Feb;58(2):278-88.
10a. Hushmendy S, Jayakumar L, Hahn A B, Bhoiwala D, Bhoiwala D L, Crawford D R. Select phytochemicals suppress human T-lymphocytes and mouse splenocytes suggesting their use in autoimmunity and transplantation. Nutr Res. 2009 Aug; 29(8): 568–578.
10b. Abeysekera I, Thomas J, Georgiadis TM, Berman AG, Hammond MA, Dria KJ, Wallace JM, Roper RJ.Differential effects of Epigallocatechin-3-gallate containing supplements on correcting skeletal defects in a Down syndrome mouse model. Mol Nutr Food Res. 2016 Apr;60(4):717-26. doi: 10.1002/mnfr.201500781. Epub 2016 Feb 11.
10c. McElyea SD, Starbuck JM, Tumbleson-Brink DM, Harrington E, Blazek JD, Ghoneima A, Kula K, Roper RJ.Influence of prenatal EGCG treatment and Dyrk1a dosage reduction on craniofacial features associated with Down syndrome.Hum Mol Genet. 2016 Sep 5. pii: ddw309.
10d. De la Torre R, De Sola S, Pons M, Duchon A, de Lagran MM, Farré M, Fitó M, Benejam B, Langohr K, Rodriguez J, Pujadas M, Bizot JC, Cuenca A, Janel N, Catuara S, Covas MI, Blehaut H, Herault Y, Delabar JM, Dierssen M. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res. 2014 Feb;58(2):278-88.
11. Li HH, He B, Peng H, Liu SQ. Effects of pyrroloquinoline quinone on proliferation and expression of c-fos, c-jun, CREB and PCNA in cultured Schwann cells. Zhonghua Zheng Xing Wai Ke Za Zhi. 2011 Jul;27(4):298-303.
12. Zhang Y, Rosenberg PA. The essential nutrient pyrroloquinoline quinone may act as a neuroprotectant by suppressing peroxynitrite formation. Eur J Neurosci. 2002 Sep;16(6):1015-24.
13. Bofill-De Ros X Santos M Vila-Casadesús M Villanueva E, Andreu N, Dierssen M, Fillat C.
Genome-wide miR-155 and miR-802 target gene identification in the hippocampus of Ts65Dn Down syndrome mouse model by miRNA sponges.BMC Genomics. 2015 Nov 6;16:907.
14. Tili E, Michaille JJ, Adair B, Alder H, Limagne E, Taccioli C, Ferracin M, Delmas D, Latruffe N, Croce CM. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis. 2010 Sep;31(9):1561-6. doi: 10.1093/carcin/bgq143. Epub 2010 Jul 9.
15. Jayasena T, Poljak A, Smythe G, Braidy N, Münch G, Sachdev P. The role of polyphenols in the modulation of sirtuins and other pathways involved in Alzheimer’s disease. Ageing Res Rev. 2013 Sep;12(4):867-83.
16. Vacca RA, Valenti D, Caccamese S, Daglia M, Braidy N, Nabavi SM. Plant polyphenols as natural drugs for the management of Down syndrome and related disorders. Neurosci Biobehav Rev. 2016 Dec;71:865-877.
17. Wang X, Song Y, Chen L, Zhuang G, Zhang J, Li M, Meng XF. Contribution of single-minded 2 to hyperglycaemia-induced neurotoxicity. Neurotoxicology. 2013 Mar;35:106-12.
18. Yanagisawa D, Ibrahim NF, Taguchi H, Morikawa S, Hirao K, Shirai N, Sogabe T, Tooyama I5.
Curcumin derivative with the substitution at C-4 position, but not curcumin, is effective against amyloid pathology in APP/PS1 mice. Neurobiol Aging. 2015 Jan;36(1):201-10.
19. Fenech M, El-Sohemy A, Cahill L, Ferguson L R, French T A C, Shyong Tai E, Milner J, Woon-Puay Koh, Lin Xie, Zucker M, Buckley M, Cosgrove L, Lockett T, Kim Y.C. Fung, and Head R. Nutrigenetics and Nutrigenomics: Viewpoints on the Current Status and Applications in Nutrition Research and Practice, J Nutrigenet Nutrigenomics. 2011 Jul; 4(2): 69–89.
20. Kussmann M, Krause L, and Siffert W. Nutrigenomics: where are we with genetic and epigenetic markers for disposition and susceptibility? Nutrition Reviews Vol. 68(Suppl. 1):S38–S47
21. Kaput J, Astley S, Renkema M, Ordovas J, and van Ommen B. Harnessing Nutrigenomics: Development of Web-based communication, databases, resources and tools. Genes & Nutrition Vol. 1, No. 1, pp. 5-12, 2006
22. Fenech M: The Genome Health Clinic and Genome Health Nutrigenomics concepts: diagnosis and nutritional treatment of genome and epigenome damage on an individual basis. Mutagenesis 2005;20:255–269.
23. 15 Fenech MF: Dietary reference values of individual micronutrients and nutriomes for genome damage prevention: current status and a road map to the future. Am J Clin Nutr 2010;91:1438S–1454S.
24. Bull C, Fenech M: Genome-health nutrigenomics and nutrigenetics: nutritional requirements or ‘nutriomes’ for chromosomal stability and telomere maintenance at the individual level. Proc Nutr Soc 2008;6
25. Garcia-Bailo B, Toguri C, Eny KM, El-Sohemy A: Genetic variation in taste and its
influence on food selection. OMICS 2009;13:69–80.
26. Simopoulos AP: Nutrigenetics/nutrigenomics. Annu Rev Public Health 2010;31:53–68.
27. Corella D, Ordovas JM: Nutrigenomics in cardiovascular medicine. Circ Cardiovasc
Genet 2009;2:637–651.
28. Trujillo E, Davis C, Milner J: Nutrigenomics, proteomics, metabolomics, and the
practice of dietetics. J Am Diet Assoc 2006;106:403–413.
29. Ferguson LR: Nutrigenomics approaches to functional foods. J Am Diet Assoc
2009;109:452–458.
30. Kaput J: Nutrigenomics research for personalized nutrition and medicine. Curr Opin
Biotechnol 2008;19:110–120.
31. Ordovas JM, Corella D: Nutritional genomics. Annu Rev Genomics Hum Genet
2004;5:71–118.
32. Fenech MF: Nutriomes and nutrient arrays – the key to personalised nutrition for DNA
damage prevention and cancer growth control. Genome Integr 2010;1:11.
